Answer: 0.374 mol CO2
Step-by-step explanation:
We can calculate the number of moles of CO, n, required to produce the specified quantity of heat. This is done by dividing the required released heat, q, by the enthalpy of formation per unit mole of the substance,
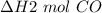 , based on the chemical equation, such that:
, based on the chemical equation, such that:
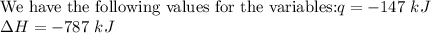

Therefore, 0.374 mol of CO2 must be reacted in order to produce 147 kJ of energy?