Given :
Mass of
 is 571.6 g per liter .
is 571.6 g per liter .
Density of solution ,
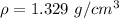 .
.
To Find :
a. Mass percentage
b. Mole fraction
c. Molality
d. molarity of H2SO4 in this solution.
Solution :
Molar mass of
 , m = 1329 g/mol .
, m = 1329 g/mol .
a ) Mass of
 contain in 1 liter is 1329 g .
contain in 1 liter is 1329 g .
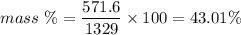
b ) Moles of
 =
=
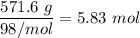 .
.
Moles of
 =
=
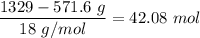 .
.
Mole fraction
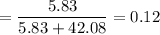 .
.
c ) Molarity of

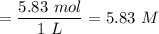 .
.
Hence , this is the required solution .