Answer:
![[A]=0.012M](https://img.qammunity.org/2021/formulas/chemistry/college/h8agojsw5tap6ldlo0ajtg28nutv23842n.png)
![[B]=0.008M](https://img.qammunity.org/2021/formulas/chemistry/college/4rix3vqluipz6xhn1ayl5sn65bjlvn2xtp.png)
Step-by-step explanation:
Hello,
In this case, given the concentration and volume of each reactant, we first compute their moles in the solution:
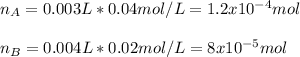
Thus, since the total volume is computed given the volume of each reactant as well as the extra water, we have:
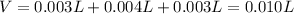
Thus, the concentrations of A and B are:
![[A]=(1.2x10^(-4)mol)/(0.010L)=0.012M](https://img.qammunity.org/2021/formulas/chemistry/college/umin45kqqbiy5t0mpmt6eaiy8o7oh9kem2.png)
![[B]=(8x10^(-5)mol)/(0.010L)=0.008M](https://img.qammunity.org/2021/formulas/chemistry/college/3w8rssaootzej4bez6q2aa92h0o56olwzc.png)
Best regards.