Given :
Electronic configuration of an electron :
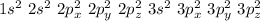 .
.
To Find :
The element having such an electronic configuration .
Solution :
We know , the sum of all the power of s and p in all the orbit gives total number of atoms in elements .
So , atomic number is :
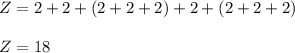
Since , the atomic number is 18 .
Therefore , the element is Argon .
Hence , this is the required solution .