Given :
A certain compound contains 4.0 g of calcium and 7.1 g of chlorine.
Its relative molecular mass is 111.
To Find :
Its empirical and molecular formulas.
Solution :
Moles of calcium ,
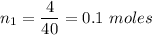 .
.
Moles of chlorine ,
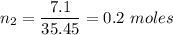 .
.
The ratio calcium and chlorine is 1 : 2 .
So , the empirical formula is
 .
.
Now , molecular mass of
 is :
is :
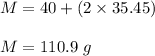
So ,
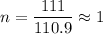
Therefore , the molecular formula is also
 .
.
Hence , this is the required solution .