Answer:
The volume in in³ is 4.19 in³
Step-by-step explanation:
To determine the volume in in³ ( cubic inches),
First, we will find the volume in cm³
From the question,
The density of aluminum = 2.70 g/cm³
Mass of the aluminum = 185.3 g
From,
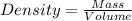
Then,
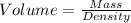
Hence,
The volume of the aluminum in cm³ is
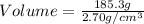
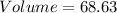 cm³
cm³
This is the volume in cm³
Now, for the volume in in³
1 in = 2.54 cm
∴ 1 in³ = 16.3871 cm³
Now,
If 16.3871 cm³ = 1 in³
Then, 68.63 cm³ =

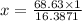
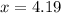 in³
in³
Hence, the volume in in³ is 4.19 in³