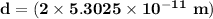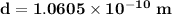Answer:
The diameter of the hydrogen
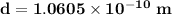
Step-by-step explanation:
From the given information:
Using the concept of Bohr's Model, the equation for the angular momentum can be expressed as:
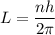
Where the generic expression for angular momentum is:
L = mvr.
replacing the value of L into the previous equation, we have:
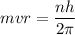
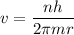 ----- (1)
----- (1)
The electron in the hydrogen atom posses an electrostatic force which gives a centripetal force.
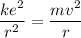 ----- (2)
----- (2)
replacing the value of v in equation (1) into (2), and taking r as the subject of the formula, we have:
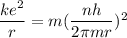
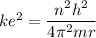
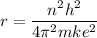
For ground-state n = 1
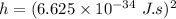
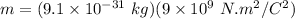
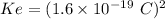
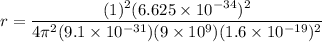
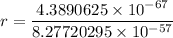
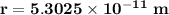
Therefore, the diameter of hydrogen d = 2r