Answer:
The correct option is E
Step-by-step explanation:
Generally the a base with the highest
 is the strongest base and from the data given
is the strongest base and from the data given
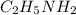 has the highest value of
has the highest value of
 so
so
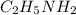 is the strongest base while
is the strongest base while
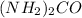 has the lowest value of
has the lowest value of
 so
so
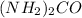 is the weakest base
is the weakest base
Generally the conjugate acid of a weak base is always a strong acid
So
For
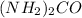 which the weakest base it conjugate acid which is
which the weakest base it conjugate acid which is
![[NH_2NH_3CO]^+](https://img.qammunity.org/2021/formulas/chemistry/college/xtrieb4qw3ietb85ho9xsh7j75d3z85e63.png) will be the strongest acid
will be the strongest acid