Answer:
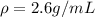
Step-by-step explanation:
Hello,
In this case, considering that the density is defined as:
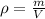
Thus, since 1 kg equals 1000 g and 1 L equal 1000 mL, the required density in g/mL turns out:
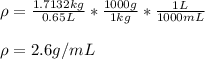
Take into account that since 0.65 L has two significant figures, the result is also shown with two significant figures.
Regards.