Answer:
The molar heat capacity at constant volume is 21.62 JK⁻¹mol⁻¹
The molar heat capacity at constant pressure is 29.93 JK⁻¹mol⁻¹
Step-by-step explanation:
We can calculate the molar heat capacity at constant pressure from
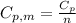
Where
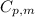 is the molar heat capacity at constant pressure
is the molar heat capacity at constant pressure
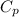 is the heat capacity at constant pressure
is the heat capacity at constant pressure
and
 is the number of moles
is the number of moles
Also
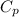 is given by
is given by
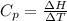
Hence,
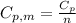 becomes
becomes
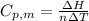
From the question,
 = 229.0 J
= 229.0 J
 = 3.00 mol
= 3.00 mol
 = 2.55 K
= 2.55 K
Hence,
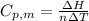 becomes
becomes
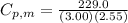
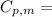 29.93 JK⁻¹mol⁻¹
29.93 JK⁻¹mol⁻¹
This is the molar heat capacity at constant pressure
For, the molar heat capacity at constant volume,
From the formula
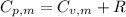
Where
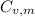 is the molar heat capacity at constant volume
is the molar heat capacity at constant volume
and
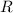 is the gas constant (
is the gas constant (
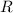 = 8.314 JK⁻¹mol⁻¹)
= 8.314 JK⁻¹mol⁻¹)
Then,
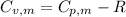
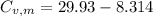
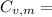 21.62 JK⁻¹mol⁻¹
21.62 JK⁻¹mol⁻¹
This is the molar heat capacity at constant volume