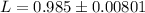Answer:
The value is
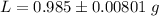
Step-by-step explanation:
From the question we are told that
The molar mass of
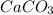 is
is
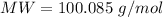
The total mass is
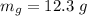
The uncertainty of the total mass is
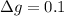
Generally the molar weight of calcium is
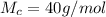
The percentage of calcium in calcite is mathematically represented as
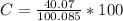
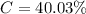
Generally the mass of each sample is mathematically represented as
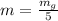
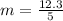
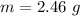
Generally mass of calcium present in a single sample is mathematically represented as
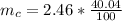
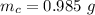
The uncertainty of mass of a single sample is mathematically represented as
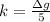
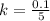
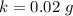
The uncertainty of mass of calcium in a single sample is mathematically represent
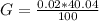
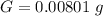
Generally the average mass of calcium in each sample is