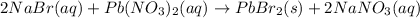Answer : The correct chemical equation is:
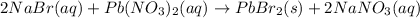
Explanation :
Balanced chemical equation : It is defined as the equation in which the atoms of individual elements present of reactant side must be equal to the product side.
According to the question, when sodium bromide reacts with lead nitrate then it reacts to give sodium nitrate and lead bromide as a products.
The balanced chemical equation will be: