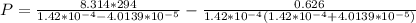Answer:
The value is

Step-by-step explanation:
From the question we are told that
The internal volume is
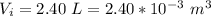
The mass of the compound contained is
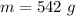
The temperature is
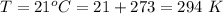
The critical temperature of silane is
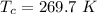
The critical pressure of silane is
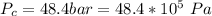
Generally the number of moles of silane inside the cylinder is mathematically represented as
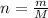
here M is the molar mass of silane with value
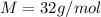
So
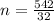
=>
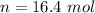
Generally the molar volume of silane in the cylinder is mathematically represented as
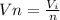
=>
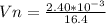
=>
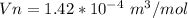
Generally from Soave-Redlich-Kwon we have that
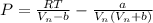
Here b is a constant which is mathematically represented as
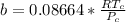
substituting
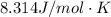 for R we have \
for R we have \
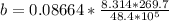
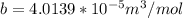
a is also a constant which is mathematically represented as
![a = 0.42748 * ((R * T_c)^2)/(P_c) * (1 + m [1-√(T_r) ])^2](https://img.qammunity.org/2021/formulas/chemistry/college/w6sf5qkuw7ehl7574krmfg09p00h2imutg.png)
Here
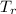 is the reduced temperature which is mathematically represented as
is the reduced temperature which is mathematically represented as
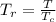
=>
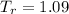
m is a constant which is mathematically represented as
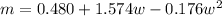
=>
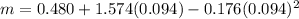
=>
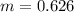
So
![a = 0.42748 * ((8.314 * 269.7)^2)/(48.4*10^(5)) * (1 + 0.626 [1-√(1.09) ])^2](https://img.qammunity.org/2021/formulas/chemistry/college/uu3he4xntshhbwaya0npeuniffo96ssh8a.png)
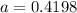
From
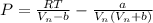 we have
we have