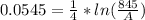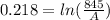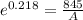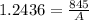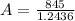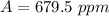Answer:
The value is
Step-by-step explanation:
From the question we are told that
The half life of
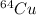 is
is
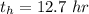
The initial concentration is
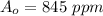
The time duration is
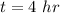
Generally the rate constant is mathematically represented as
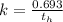
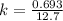
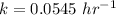
This rate constant is also mathematically represented as
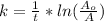
Here A is the remaining concentration after t
So