Answer:
*
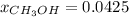
*
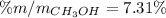
*
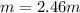
Step-by-step explanation:
Hello,
In this case, for the mole fraction of methanol we use the formula:
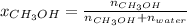
Thus, we compute the moles of both water (molar mass 18 g/mol) and methanol (molar mass 32 g/mol):
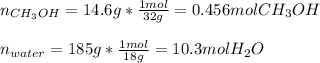
Hence, mole fraction is:
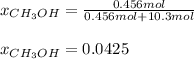
Next, mass percent is:
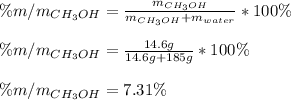
And the molality, considering the mass of water in kg (0.185 kg):
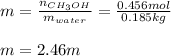
Regards.