Answer:
The change in energy of the gas during the process is
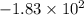 joules.
joules.
Step-by-step explanation:
We can represent this process by the First Law of Thermodynamics, in which gas does work on its surroundings and absorbs heat from there to describe its change in energy. In other words:
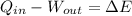
Where:
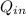 - Heat absorbed by the gas, measured in joules.
- Heat absorbed by the gas, measured in joules.
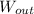 - Work done by the gas, measured in joules.
- Work done by the gas, measured in joules.
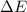 - Change in energy, measured in joules.
- Change in energy, measured in joules.
If we know that
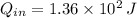 and
and
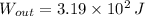 , the change in energy of the gas is:
, the change in energy of the gas is:
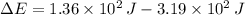
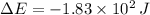
The change in energy of the gas during the process is
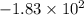 joules.
joules.