Answer:
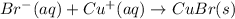
Step-by-step explanation:
Hello,
In this case, the molecular chemical reaction is written as:
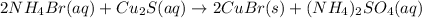
In such a way, considering the net ionic notation and the solid yielded copper (I) bromide, we write:
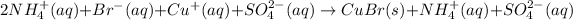
Whereas ammonium and sulfate ions are spectator ions, therefore the net ionic equation is:
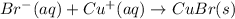
Regards.