Answer:
609 grams
Step-by-step explanation:
Given that,
The density of silver is 10.5 g/mL
We need to find the weight of the silver if we have 58 mL of silver.
It is based on the concept of the definition of density. It is equal to mass per unit volume. So,
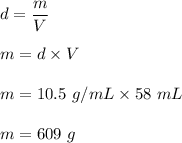
Hence, the mass of the silver is 609 grams.