Answer:
15 g
Step-by-step explanation:
The density of an object can be found using the following formula:
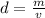
We want to find the mass, so we must rearrange the formula for
 . Multiply both sides of the formula by
. Multiply both sides of the formula by
 .
.
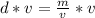
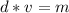
The mass can be found by multiplying the density and volume.
The density of the object is 1.5 g/mL and the volume is 10 mL.
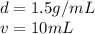
Substitute the values into the formula.
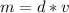
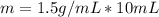
Multiply. When multiplying, the milliliters, or mL will cancel out.
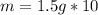
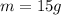
The mass of the object is 15 grams.