Answer:
A)
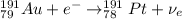
B)
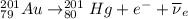
C)
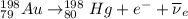
D)
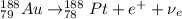
Step-by-step explanation:
A) The reaction of electron capture is:
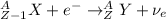
Where:
A: is the mass number = n + Z
Z: is the number of protons
n: is the number of neutrons
In electron capture reaction a proton of the atom is converted into a neutron and an electron neutrino (
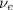 ) is emitted.
) is emitted.
Hence, for the Gold-191 we have:
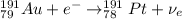
B) The nuclear reaction of the decay of Au-201 to Hg-201 is:
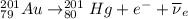
The above reaction is a beta decay reaction, in which a positron and electron antineutrino (
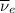 ) are emitted from the Au-201 nucleus and a neutron is converted to a proton (n-1 and Z+1; A remains constant).
) are emitted from the Au-201 nucleus and a neutron is converted to a proton (n-1 and Z+1; A remains constant).
C) The nuclear reaction of beta emission Au-198 is:
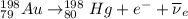
Same as above, a beta emission produces the emission of a positron and electron antineutrino (
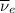 ) and the conversion of a neutron into a proton.
) and the conversion of a neutron into a proton.
D) Gold-188 decays by positron emission:
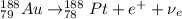
In a positron emission, we have that a proton of Au-188 is converted into a neutron and a positron (e⁺) and an electron neutrino (
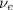 ) are released.
) are released.
I hope it helps you!