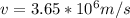Answer:
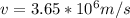
Step-by-step explanation:
Given
de Broglie wavelength = 0.20nm
Required
Determine the speed (v)
The speed is calculated using the following formula;
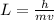
Where
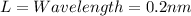
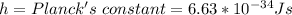
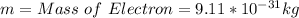
Substitute these values in the above formula
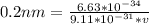
-----------------------------------------------------
Convert 0.2nm to metre (m)
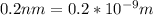
-----------------------------------------------------
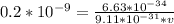
Multiply both sides by v
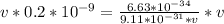
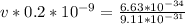
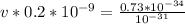
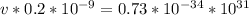
Apply law of indices
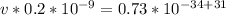
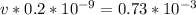
Divide both sides by
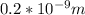
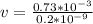
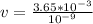
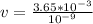
Apply law of indices
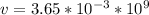
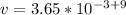
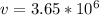
Hence;
The velocity is