Answer:
1493 balloons.
Step-by-step explanation:
Hello,
In this case, we first compute the total moles by temperature by using the ideal gas equation as shown below:
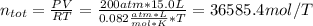
Moreover, since the tank cannot be emptied, we compute the moles that are not transferred:
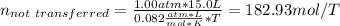
Now, we compute the moles that are actually transferred:
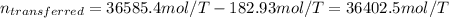
Next, since each balloon is filled up to 1.00 atm within a volume of 2.00 L, the moles per balloon are:
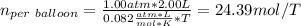
Then, the number of balloons are:
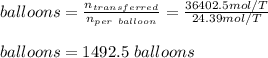
Which is more accurately 1493 balloons.
Regards.