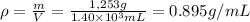Answer:
0.895 g/mL
Step-by-step explanation:
Step 1: Given data
Mass of liquid Z (m): 2.763 lb
Volume of liquid Z (V): 5.93 cups
Step 2: Convert "m" to grams
We will use the relationship 1 lb = 453.59 g.
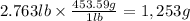
Step 3: Convert "V" to milliliters
We will use the relationship 1 cup = 236.59 mL.
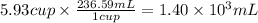
Step 4: Calculate the density of the liquid Z
The density (ρ) of the liquid Z is equal to its mass divided by its volume.