Answer:
The answer is
0.267 moles
Step-by-step explanation:
To find the number of moles of NaCl we must first find it's molecular mass M after that we use the formula
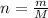
where
n is the number of moles
m is the mass
M is the molecular mass
From the question
m of NaCl = 15.6 g
M( NaCl ) = 22.99 + 35.45 = 58.44 g/mol
Substitute the values into the above formula and solve
That's

We have the final answer as
0.267 moles
Hope this helps you