Answer:
the reaction rate of 1,000 k is 17.28 m/s
Step-by-step explanation:
The computation of the reaction rate in case when NO is increased to 0.12 M is shown below:
According to the question, data provided is as follows
NO = 0.12M
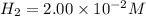
Based on the above information
Reaction rate is
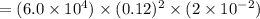
= 17.28 M/s
Hence, the reaction rate of 1,000 k is 17.28 m/s and the same is to be considered