Answer:
The percent error is 23%
Step-by-step explanation:
To find the percent error for the experiment carried out,
Percent error is given by the formula below
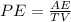 ×
×
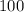 %
%
Where PE is the percent error
AE is the Absolute error
TV is the Theoretical value
Also, Absolute error (AE) can be determined from
Absolute error (AE) = /Theoretical value(TV) - Experimental value (EV)/
Now, from the question,
Experimental value (EV) = 1.23 g/mL
Theoretical value (TV) = 1.00 g/mL
From,
Absolute error (AE) = /Theoretical value(TV) - Experimental value (EV)/
Absolute error (AE) = /1.00 g/mL - 1.23 g/mL/
Absolute error (AE) = / - 0.23 g/mL/
Absolute error (AE) = 0.23 g/mL
This is the Absolute error
Now, for the Percent error
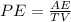 ×
×
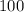 %
%
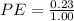 ×
×
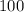 %
%
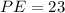 %
%
Hence, the percent error (PE) is 23%