Answer:
30, 50
Step-by-step explanation:
Hello,
In this since an element's atomic number is equal to the number of protons in its atom, we can infer that selenium's atomic number is 30. Moreover, due to the fact the the neutrons are equal to the atomic mass minus the atomic number or the number of protons, by knowing the number of neutrons we compute the atomic as follows:
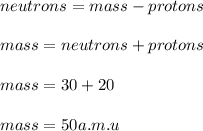
Thus, answer is 30, 50.
Best regards.