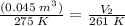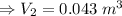Hi! the english version of the given question is "A helium balloon is inflated to the volume of 0.045 m3 at a temperature of 2 ° C. If the balloon is cooled to -12 ° C. What will its new volume be? Consider that the pressure does not vary". The answer is given in english.
Answer:
The new volume of balloon is
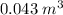 .
.
Step-by-step explanation:
Let's assume the helium gas inside the balloon behaves ideally.
The total number of moles of helium gas inside the balloon remains constant.
Applying combined gas law, we get:
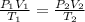
Where:
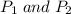 are the initial and final pressure of the balloon respectively.
are the initial and final pressure of the balloon respectively.
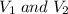 are the initial and final volume of the balloon respectively.
are the initial and final volume of the balloon respectively.
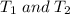 are the initial and final temperature in the kelvin scale respectively.
are the initial and final temperature in the kelvin scale respectively.
Given:
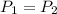
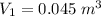
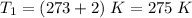
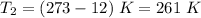
Substituting the above values, we get: