Answer:
The metallurgist should use 3 ounces of the 30 % copper alloy and 9 ounces of the 50 % copper alloy to make 12 ounces of 45 % copper alloy.
Explanation:
The ounce is a mass unit, as we notice that the metallurgist wants to make 12 ounces of an alloy containing 45 % copper by mixing two metal with different copper proportions. We can use the following two equations:
Alloys
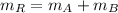 (Eq. 1)
(Eq. 1)
Copper
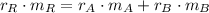 (Eq. 2)
(Eq. 2)
Where:
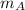 - Mass of the 30 % copper alloy, measured in ounces.
- Mass of the 30 % copper alloy, measured in ounces.
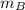 - Mass of the 50 % copper alloy, measured in ounces.
- Mass of the 50 % copper alloy, measured in ounces.
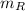 - Mass of the 45 % copper alloy, measured in ounces.
- Mass of the 45 % copper alloy, measured in ounces.
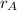 - Proportion of copper in the 30 % copper alloy, dimensionless.
- Proportion of copper in the 30 % copper alloy, dimensionless.
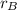 - Proportion of copper in the 50 % copper alloy, dimensionless.
- Proportion of copper in the 50 % copper alloy, dimensionless.
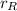 - Proportion of copper in the 45 % copper alloy, dimensionless.
- Proportion of copper in the 45 % copper alloy, dimensionless.
Now, the mass of the 50 % copper alloy is cleared in Eq. 1 and eliminated in Eq. 2:
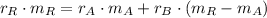
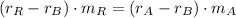
And we clear and calculate the mass of the 30 % copper alloy:
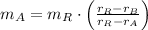
If we know that
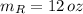 ,
,
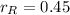 ,
,
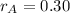 and
and
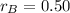 , the mass of the 30 % copper alloy:
, the mass of the 30 % copper alloy:
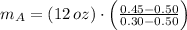
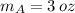
And the mass of the 50 % copper alloy is:
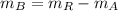
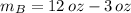
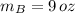
The metallurgist should use 3 ounces of the 30 % copper alloy and 9 ounces of the 50 % copper alloy to make 12 ounces of 45 % copper alloy.