Answer:
Z=22.70
Step-by-step explanation:
It is given that,
An element Z that has two naturally occurring isotopes with the following percent abundances as follows :
The isotope with a mass number 22 is 65.0% abundant; the isotope with a mass number 24 is 35.0% abundant.
The average atomic mass for element Z is given by :
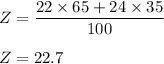
So, the average atomic mass for element Z is 22.70.