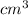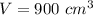Answer:
The value is
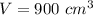
Step-by-step explanation:
From the question we are told that
The power output is
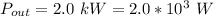
The mass of the steel is
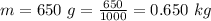
The temperature of the water is
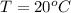
The time take is
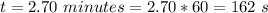
Generally the quantity of heat energy given out by the electric stove is mathematically represented as
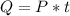
=>
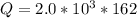
=>
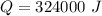
This energy can also be mathematically represented as
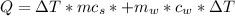
Here
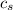 is the specific heat of stainless steel with value
is the specific heat of stainless steel with value
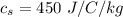
tex]c_s[/tex] is the specific heat of water with value
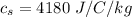
m_w is the mass of water which is mathematically represented as
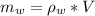
=>
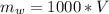
So
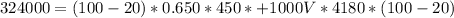
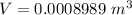
converting to