Step-by-step explanation:
1) Complete combustion of propane gives carbon dioxide gas and water vapors.
The balanced chemical equation is given as:
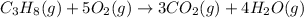
Number of carbon atoms on reactant side = 3
Number of hydrogen atoms on reactant side = 8
Number of oxygen atoms on reactant side = 10
Number of carbon atoms on the product side = 3
Number of hydrogen atoms on the product side = 8
Number of oxygen atoms on the product side = 10
On both the sides numbers, all elements remained the same which indicates that matter remains conserved during the course of a chemical reaction.
2) Incomplete combustion of propane gives carbon dioxide gas, carbon monoxide gas, and water vapors.
The balanced chemical equation is given as:
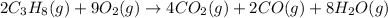
Number of carbon atoms on reactant side = 6
Number of hydrogen atoms on reactant side = 16
Number of oxygen atoms on reactant side = 18
Number of carbon atoms on the product side = 6
Number of hydrogen atoms on the product side = 16
Number of oxygen atoms on the product side = 18
On both the sides numbers, all elements remained the same which indicates that matter remains conserved during the course of a chemical reaction.