Answer:
The number of atoms is 6.83 grams of potassium is
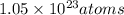 .
.
Step-by-step explanation:
Mass of the potassium element = 6.83 g
Atomic mass of potassium = 39.10 g/mol
Moles of potassium =
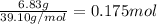
According to mole concept :
1 mole =
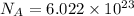 atoms/ions/ molecules
atoms/ions/ molecules
Total number of atoms of potassium in 0.175 moles:
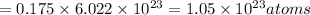
So, number of atoms is 6.83 grams of potassium is
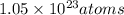 .
.