Answer:
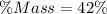
Step-by-step explanation:
Given
Sugar = 56g
Tea = 78g
Required
Determine the mass percent of the sugar
First we need to calculate the total mass
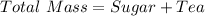
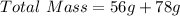
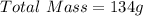
The percentage mass is then calculated as thus
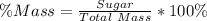
Substitute 56 and 134 for Sugar and Total mass respectively
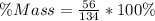
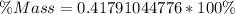
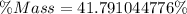
Approximate
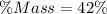
Hence, the percentage mass of sugar is 42%