Answer:
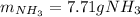
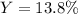
Step-by-step explanation:
Hello,
In this case, given the chemical reaction and the initial amounts, we first identify the limiting reactant by verifying the reactant yielding the smallest amount of product:
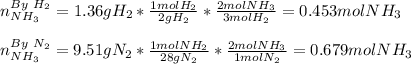
Thus, since the hydrogen yields the least amount of ammonia, it is the limiting reactant, which means the theoretical yield of ammonia is:
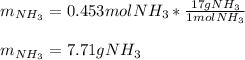
Finally, the percent yield for a 1.06-g actually yielded amount turns out:
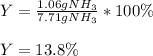
Best regards.