Answer:
the additional concentration of nitrogen = 0.04748 mg/L
Step-by-step explanation:
From the given information:
The mass of the nitrogen added to the field per year = 10% of 1300.0 kg
=
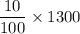
= 130 kg
The mass of the nitrogen that is being also washed into the river
= 15% of 130 kg
=
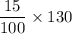
= 15 × 1.3 kg
= 19.5 kg /year
Converting kg/year into milligram per seconds; we have:
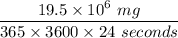
= 0.61834 mg/seconds
If the river flows at an average rate of 0.460 cubic feet
Let's convert the cubic feet into liters
we all know that;
1 ft = 0.33 meter
1 cubic feet = 28.31 liters
∴ 0.460 cubic feet = 0.460 × 28.31
13.0226 liters
Finally, the additional concentration of nitrogen =
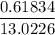
the additional concentration of nitrogen = 0.04748 mg/L