Given :
A 10.99 g sample of NaBr contains 22.34% Na by mass.
To Find :
How many grams of sodium does a 9.77g sample of sodium bromine contain.
Solution :
By law of constant composition , in any given chemical compound, the elements always combine in the same proportion with each other.
Therefore , percentage of Na by mass in NaBr will be same for every amount .
Percentage of Na in 9.77 g NaBr is 22.34 % too .
Gram of Na =
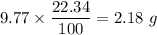 .
.
Hence , this is the required solution .