Answer:
Approximately
 .
.
Step-by-step explanation:
Start by finding the balanced equation for the reaction between
 and
and
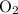 .
.
When
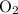 is in excess,
is in excess,
 reacts with
reacts with
 to produce
to produce
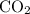 .
.
 .
.
Assume that the coefficient of
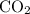 is
is
 . (
. (
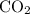 is the only product of this reaction.)
is the only product of this reaction.)
 .
.
Apply the conservation of atoms to find the coefficient of the other two species.
 .
.
 .
.
Multiply all coefficients in this equation by
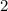 to eliminate the fraction.
to eliminate the fraction.
 .
.
Note the ratio between the coefficient of
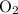 and
and
 in this balanced equation:
in this balanced equation:
 .
.
Assume that both
 and
and
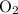 behave like ideal gases. Under the same temperature and pressure, the ratio between the volume of these two gases will match the ratio between the quantity of their particles:
behave like ideal gases. Under the same temperature and pressure, the ratio between the volume of these two gases will match the ratio between the quantity of their particles:
 .
.
In other words, in this reaction, every one unit volume of
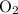 will react with two unit volume of
will react with two unit volume of
 .
.
 .
.
Under these assumptions:
 .
.
The question stated that when measured by volume, one-fifth of the air is oxygen. That is:
 .
.
Rearrange to obtain:
 .
.
Therefore:
