Answer:
3.3557047 mL
Step-by-step explanation:
The density can be found using the following formula:
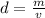
Let's rearrange the formula to find the volume,
 .
.
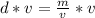
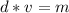
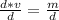
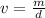
The volume can be found by dividing the mass by the density. The mass of the chloroform is 5 grams and the density is 1.49 grams per milliliter. Therefore,
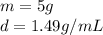
Substitute the values into the formula.
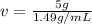
Divide. When we divide, the grams, or g, in the numerator and denominator will cancel out.
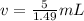
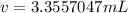
The volume of 5 grams of chloroform is 3.3557047 milliliters