Answer:
607 ppm
Step-by-step explanation:
In this case we can start with the ppm formula:
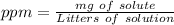
If we have a solution of 0.0320 M, we can say that in 1 L we have 0.032 mol of
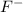 , because the molarity formula is:
, because the molarity formula is:
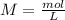
In other words:
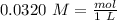
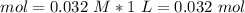
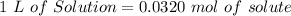
If we use the atomic mass of
 (19 g/mol) we can convert from mol to g:
(19 g/mol) we can convert from mol to g:
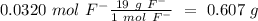
Now we can convert from g to mg (1 g= 1000 mg), so:
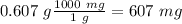
Finally we can divide by 1 L to find the ppm:
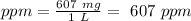
We will have a concentration of 607 ppm.
I hope it helps!