Answer:
freezing point (°C) of the solution = - 3.34° C
Step-by-step explanation:
From the given information:
The freezing point (°C) of a solution can be prepared by using the formula:
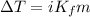
where;
i = vant Hoff factor
the vant Hoff factor is the totality of the number of ions in the solution
Since there are 1 calcium ion and 2 nitrate ions present in Ca(NO3)2, the vant Hoff factor = 3
 = 1.86 °C/m
= 1.86 °C/m
m = molality of the solution and it can be determined by using the formula
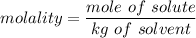
which can now be re-written as :
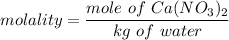
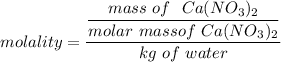
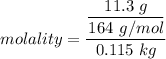
molality = 0.599 m
∴
The freezing point (°C) of a solution can be prepared by using the formula:
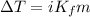
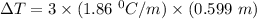
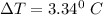
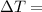 the freezing point of water - freezing point of the solution
the freezing point of water - freezing point of the solution
3.34° C = 0° C - freezing point of the solution
freezing point (°C) of the solution = 0° C - 3.34° C
freezing point (°C) of the solution = - 3.34° C