Answer:
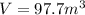
Step-by-step explanation:
Hello,
In this case, considering that the density is defined in terms of mass and volume as follows:
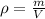
Due to the fact that 100 tons of water were displaced, whose value in grams is:
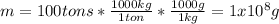
Thus the displaced volume in cubic meters was:
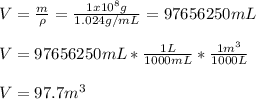
Regards.