Answer:
Non-polar compounds:
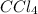 ,
,
 ,
,

Polar compounds:
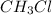 ,
,

Step-by-step explanation:
For this question, we must start with the Lewis structure for each molecule and then we can do their respective analysis:
-)
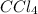
In this case, we have 4 equal atoms attached to the central atom. Therefore, we have the same magnitude of electronegativity. Chlorine atoms have different and opposite directions. Therefore due to the orientation the dipole moments cancel and the net dipole moment will be zero and the molecule will be non-polar.
-)

In this case, we have a linear structure in which the magnitude of the dipole moment is the same, but the direction is the opposite. Therefore the dipole moments are canceled and the molecule will be non-polar.
-)

In this case, we also have a linear structure in which the magnitude of the dipole moment is the same, but the direction is the opposite. Therefore the dipole moments are canceled and the molecule will be non-polar.
-)
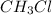
For this molecule, we have a different atom. The hydrogen atom, therefore the magnitude of one of the atoms attached to the central atom is different and the magnitude of the net dipole moment will be different from zero and the molecule will be polar.
-)

For this molecule, due to the structure of the molecule, the dipole moments of oxygens will not have a totally opposite configuration. Therefore, the net dipole moment will be different from zero and the molecule will be polar.
See figure 1 to further explanations
I hope it helps!