Given :
Energy , E = 330 J .
Initial temperature ,
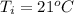 .
.
Final temperature ,
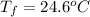 .
.
Mass of benzene , m = 24.6 g .
To Find :
The molar hear capacity of benzene at constant pressure .
Solution :
Molecular mass of benzene , M = 78 g/mol .
Number of moles of benzene :
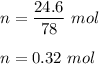
Energy required is given by :
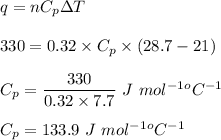
Hence , this is the required solution .