Answer:
pKa = 9
Step-by-step explanation:
In this case, we have to remember the meaning of "p". When we use "p" in any calculation we have to apply the minus ten based logarithm, so:

For example, when we use the "pH" value. We have to apply the minus ten based logarithm of the concentration of the hydronium ion:
![pH~=~-Log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/j3i3zy8uuheep8gwmexx0osobnzs9e8h77.png)
So, if we have wanted to calculate the pKa value. We have to apply the minus ten based logarithm of the Ka value, so:

Now we can plug the value into the equation:
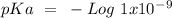
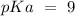
The pKa value is 9.
I hope it helps!