Answer:
So we know that: E = h*(frequency)
but for this equation, we need energy in joules
so since 1eV is 1.6 *
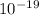
we will multiply the energy in eV to 1.6 * 10^-19
so energy in joules:
20000 * 1.6 * 10^-19
3.2 *
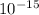 j
j
Now, finding the frequency:
3.2 * 10^-15 = 6.6 *
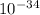 * frequency
* frequency
frequency = (3.2 *
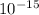 ) / (6.6 *
) / (6.6 *
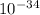 )
)
frequency = 4.8 *
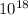 Hz
Hz
Finally, finding the wavelength:
we will use a different formula here,
frequency * wavelength = speed of light
wavelength = speed of light / frequency
wavelength = 3 *
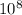 / 4.8 *
/ 4.8 *
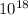
wavelength = 3 *
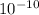 / 4.8
/ 4.8
wavelength = 0.625 *
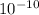
wavelength = 6.25 *
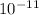 m
m
frequency = 4.8 *
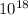 Hz
Hz