Answer:
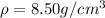
Step-by-step explanation:
Hello,
In this case, due to the volume difference caused by the addition of the metal, one could notice that the volume of the metal is:
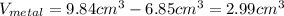
In such a way, given the mathematical definition of density, it turns out:
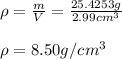
Regards.