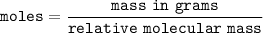Answer:
Step-by-step explanation:
A mole is the amount of substance that contains as many elementary entities as there are carbon atoms in 12 grams of carbon -12 isotopes i.e. it is the amount of a substance that posses the same number of particles as the number of atoms in exactly 12g of carbon is 12.
The number of atoms in a mole is known to be the Avogadro Number which is equal to 6.02 × 10²³. Thus, a mole of any substance is that amount of the substance which contains 6.02 × 10²³ particles of that substance. The definition of the mole is also given by the relationship: