Answer:
The current mass fraction of
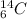 should be approximately 68.8 percent.
should be approximately 68.8 percent.
Step-by-step explanation:
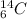 is a radioactive isotope with a halflife of 5568 years. The decay of any radioisotope is modelled after the following ordinary differential equation:
is a radioactive isotope with a halflife of 5568 years. The decay of any radioisotope is modelled after the following ordinary differential equation:
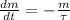
Where:
 - Current mass of the isotope, measured in grams.
- Current mass of the isotope, measured in grams.
 - Time constant, measured in years.
- Time constant, measured in years.
The solution of this equation is of the form:
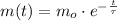
Where:
 - Time, measured in years.
- Time, measured in years.
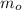 - Initial mass of the isotope, measured in grams.
- Initial mass of the isotope, measured in grams.
The time constant can be found as a function of halflife (
 ):
):
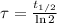
If
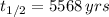 and
and
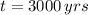 , the mass fraction of
, the mass fraction of
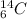 is:
is:
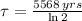
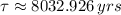
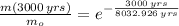
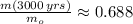
The current mass fraction of
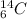 should be approximately 68.8 percent.
should be approximately 68.8 percent.