Answer:
8.76M
Step-by-step explanation:
Given that
Mass from the density = 1141g
According to the given situation the computation of molarity of the solution is shown below:-
we will took HCL solution which is 1000mL
HCl = 28% by mass
So,
Mass of HCl in 1-litre solution is
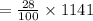
Which gives the result of molar mass HCI is
= 319.48g /mol
Now,
Molarity is
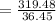
Which gives results of molarity is
= 8.76M